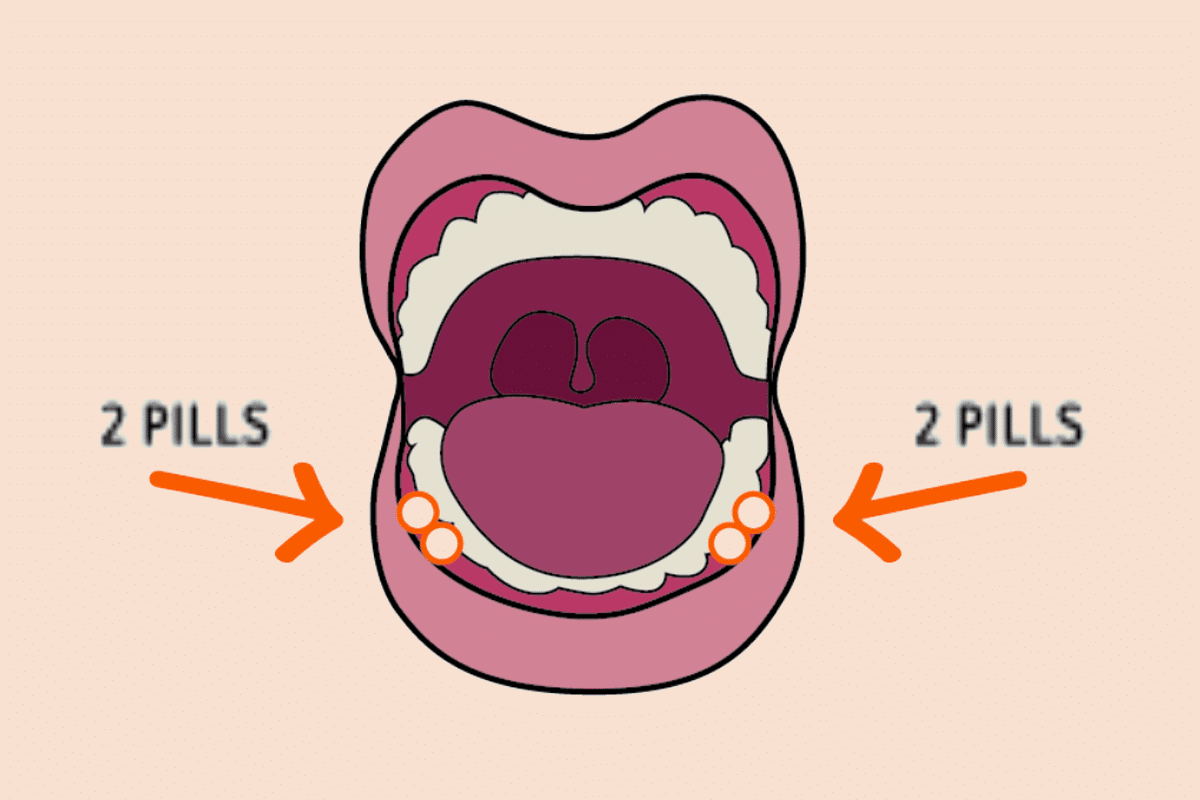
Image: Put two pills under the tongue on each side of your mouth, between your gums and cheeks.
Governments worldwide have an obligation to ensure that reproductive health information, supplies and services are available, accessible, acceptable and of good quality. The WHO advises that everyone has the right to access safe and effective abortion care. However, in 2023, there remain many factors working against people’s access to such care. This Editorial discusses two key areas which continue to restrict women’s rights to obtain safe and effective medical abortion.
Medicines licensing
Misoprostol was initially licensed in the 1980s solely for the treatment of peptic ulceration and its use in reproductive health since then has been widely off-label. It has become the prostaglandin of choice for early medical abortion (EMA) (abortion at less than 10 weeks’ gestation) even though the product licence still does not include this indication.
In contrast, mifepristone’s sole indication is for termination of pregnancy. Since mifepristone was registered in France in 1988, it has become clear that adding mifepristone to misoprostol significantly improves efficacy. Used alone, misoprostol will abort about 80% of early pregnancies, although this may take some time and may involve multiple doses.6 A combined mifepristone/misoprostol regimen results in complete abortion in 95–98% of cases. Despite this evidence, and the fact that mifepristone is on the WHO Essential Medicines List, around half (98/193) of countries have not licensed it for EMA, including Brazil, Egypt, Indonesia, Pakistan and the Philippines. Therefore, nearly 200 million women of childbearing age in these five countries cannot access mifepristone through health services. Regulators who hesitate or decline marketing authorisation of mifepristone are not making their decisions based on scientific evidence.
The safety of mifepristone is now well established after more than three decades of use, approval in 94 countries and more than 100 studies that have demonstrated its safety.
SOURCE: BMJ Sexual & Reproductive Health, by Sam Rowlands, Mira Harrison-Woolrych, 28 August 2023. (Not open access) ; VISUAL: Abortion pill instructions, Shore Centre Clinic, 6 September 2022



