
When there are no abortion laws: A case study of Canada
by Dorothy Shaw, Wendy V Norman
Best Practice & Research Clinical Obstetrics & Gynaecology 2020;62(January):49-62
Highlights
– Canada’s 30-year experience may guide others considering decriminalizing abortion.
– Regulating abortion as a healthcare service has not led to higher abortion rates.
– Canada’s geography and population density create specific access challenges.
– Average gestational age at abortion is decreasing as access to services increases.
– As medical abortion increases, training and access for second trimester abortion are concerning.
Abstract
Canada decriminalized abortion, uniquely in the world, 30 years ago. We present the timeline of relevant Canadian legal, political, and policy events before and since decriminalization. We assess implications for clinical care, health service and systems decisions, health policy, and the epidemiology of abortion in the absence of criminal legislation. As the criminal abortion law was struck down, dozens of similar private member’s bills, and one government bill, have been proposed, but none were passed. Key findings include that initially Canadian provinces attempted to provide restrictive regulations and legislation, all of which have been revoked and largely replaced with supportive policies that improve equitable, accessible, state-provided abortion service. Abortion rates have been stable over 30 years since decriminalization, and a falling proportion of abortions occur late in the second trimester. Canada demonstrates that abortion care can safely and effectively be regulated as a normal component of usual medical care.
+++
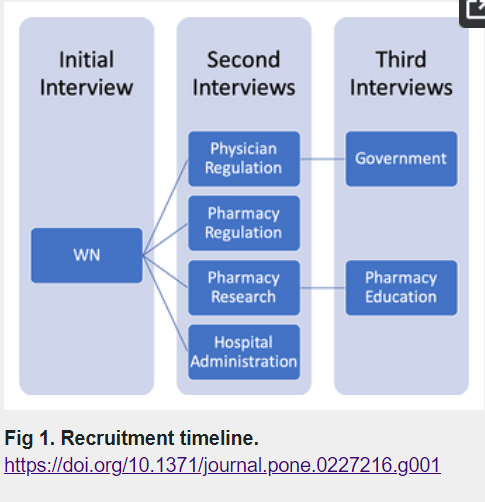
Leadership for success in transforming medical abortion policy in Canada
by Brigid Dineley, Sarah Munro, Wendy V Norman
PLOS One. 8 January 2020.
Abstract
Objectives: Mifepristone was approved for use in medical abortion by Health Canada in 2015. Approval was accompanied by regulations that prohibited pharmacist dispensing of the medication. Reproductive health advocates in Canada recognized this regulation would limit access to medical abortion and successfully worked to have this regulation removed in 2017. The purpose of this study was to assess the leadership involved in changing these regulations so that the success may be replicated by other groups advocating for health policy change.
Methods: This study involved a mixed methods instrumental design in the context of British Columbia, Canada. Our data collection included: a) interviews with seven key individuals, representing the organizations that worked in concert for change to Canadian mifepristone regulations, and b) document analysis of press articles, correspondence, briefing notes, and meeting minutes. We conducted a thematic analysis of transcripts of audio-recorded interviews. We identified strengths and weaknesses of the team dynamic using the Develop Coalitions, Achieve Results and Systems Transformation domains of the LEADS Framework.
Results: Our analysis of participant interviews indicates that autonomy, shared values, and clarity in communication were integral to the success of the group’s work. Analysis using the LEADS Framework showed that individuals possessed many of the capabilities identified as being necessary for successful health policy leadership. A lack of post-project assessment was identified as a possible limitation and could be incorporated in future work to strengthen dynamics especially when a desired outcome is not achieved. Document analysis provided a clear time-line of the work completed and suggested that strong communication between team members was another key to success.
Conclusions: The results of our analysis of the interviews and documents provide valuable insight into the workings of a successful group committed to a common goal. The existing collegial and trusting relationships between key stakeholders allowed for interdisciplinary collaboration, rapid mobilization, and identification of issues that facilitated successful Canadian global-first deregulation of mifepristone dispensing.
+++
Advancing reproductive health through policy-engaged research in abortion care
by Sarah B Munro, Sheila Dunn, Edith R Guilbert, Wendy V Norman
Seminars in Reproductive Medicine 2022; 40(05/06): 268-76. DOI: 10.1055/s-0042-1760213
Abstract
Mifepristone medication abortion was first approved in China and France more than 30 years ago and is now used in more than 60 countries worldwide. It is a highly safe and effective method that has the potential to increase population access to abortion in early pregnancy, closer to home. In both Canada and the United States, the initial regulations for distribution, prescribing, and dispensing of mifepristone were highly restricted. However, in Canada, where mifepristone was made available in 2017, most restrictions on the medication were removed in the first year of its availability. The Canadian regulation of mifepristone as a normal prescription makes access possible in community primary care through a physician or nurse practitioner prescription, which any pharmacist can dispense. In this approach, people decide when and where to take their medication. We explore how policy-maker-engaged research advanced reproductive health policy and facilitated this rapid change in Canada. We discuss the implications of these policy advances for self-management of abortion and demonstrate how in Canada patients “self-manage” components of the abortion process within a supportive health care system.



